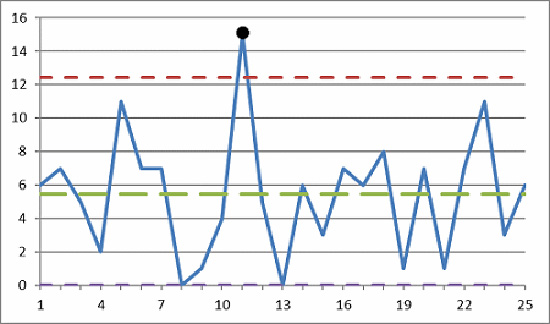
Identifying outlier data points using visual and analytical techniques is especially important for proper process validation, control, and monitoring in the FDA regulated industries.
A confirmed outlier data point should never be deleted; however, it should be excluded from any subsequent calculations. Best practice is to provide the rationale and the method used to determine if the suspect point(s) is/are indeed outliers and document the cause(s) for the outlier(s).
We need to identify and consider excluding outlier data points:
- to provide a realistic picture of a process
- to provide meaningful control limits
- to prevent “bonus” statistical control limits
- to ensure actions are taken only when appropriate,
What are your thoughts on outliers and how they affect data?
See full article at https://www.pharmaceuticalonline.com/doc/identifying-outliers-in-process-data-using-visual-and-analytical-techniques-0001