By Mark Durivage, Quality Systems Compliance LLC
Every organization that is regulated by the FDA must consider sources of contamination and crosscontamination in their manufacturing operations. A robust personnel hygiene program is an important element for good manufacturing practices (GMPs) necessary to prevent contamination and cross-contamination, in order to ensure the safety of medicinal products.
Requirements And Background
Pharmaceutical and medical device regulations, standards, and guidances provide direction and lay out the framework for successfully implementing, maintaining, and sustaining an effective robust system of good manufacturing practice (GMPs) to prevent contamination and cross-contamination.
Specifically, 21 CFR Part 211 Current Good Manufacturing Practice for Finished
Pharmaceuticals, 21 CFR 820 Quality System Regulation, and ICH Q7 Good
Manufacturing Practice Guide for Active Pharmaceutical Ingredients include requirements that address personnel hygiene.
21 CFR Part 820 Quality System Regulation
Sec. 820.70 Production and process controls.
(e) Contamination control. Each manufacturer shall establish and maintain procedures to prevent contamination of equipment or product by substances that
could reasonably be expected to have an adverse effect on product quality.
21 CFR Part 211 Current Good Manufacturing Practice for Finished
Pharmaceuticals
Sec. 211.28 Personnel responsibilities.
(a) Personnel engaged in the manufacture, processing, packing, or holding of a drug product shall wear clean clothing appropriate for the duties they perform.
Protective apparel, such as head, face, hand, and arm coverings, shall be worn as necessary to protect drug products from contamination.
(b) Personnel shall practice good sanitation and health habits
(c) Only personnel authorized by supervisory personnel shall enter those areas of the buildings and facilities designated as limited-access areas.
(d) Any person shown at any time (either by medical examination or supervisory observation) to have an apparent illness or open lesions that may adversely affect the safety or quality of drug products shall be excluded from direct contact with components, drug product containers, closures, in-process materials, and drug products until the condition is corrected or determined by competent medical personnel not to jeopardize the safety or quality of drug products. All personnel shall be instructed to report to supervisory personnel any health conditions that may have an adverse effect on drug products.
ICH Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients
3.2 Personnel Hygiene
3.20 Personnel should practice good sanitation and health habits.
3.21 Personnel should wear clean clothing suitable for the manufacturing activity with which they are involved and this clothing should be changed when appropriate. Additional protective apparel, such as head, face, hand, and arm coverings, should be worn when necessary, to protect intermediates and APIs from contamination.
3.22 Personnel should avoid direct contact with intermediates or APIs.
3.23 Smoking, eating, drinking, chewing and the storage of food should be restricted to certain designated areas separate from the manufacturing areas.
Sources Of Contamination
Sources of contamination can include biological, chemical, physical, and radiological. For an organization to successfully implement, maintain, and sustain an effective personnel hygiene program, the possible sources of contamination related to people must be identified and then mitigated. Hazard analysis and critical control point (HACCP) is a risk management tool to identify sources of contamination and use that information to protect products from intentional and unintentional contamination, from raw materials through finished products. Although HACCP has its roots in the food industry, the principles can and have been adapted to other industries including medical devices, cosmetics, and pharmaceuticals.
A process flow diagram is a good way to help analyze where potential sources of contamination or cross-contamination can affect the safety and quality of medicinal products. Figure 1 is an example process flow diagram.
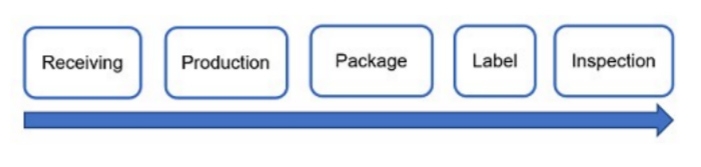
Figure 1: Example process flow diagram
Once a process flow diagram has been constructed, the HACCP plan can be developed for each step in the process. For the purpose of this article, we will strictly focus on sources of contamination (biological, chemical, physical, and radiological) related to personnel hygiene. See Figure 2 for an example HACCP for production related to personnel hygiene.
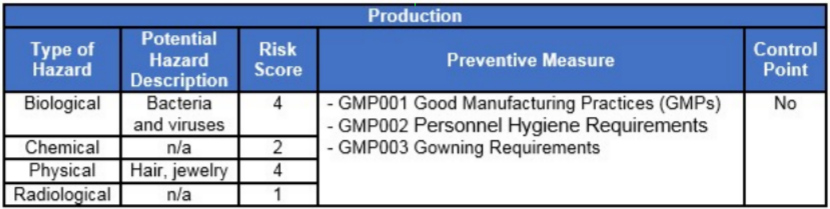
Figure 2: Example HACCP for production
In this example, the HACCP team considered the preventive measures (prerequisite program procedures) sufficient. Therefore, additional controls were not be required.
Personnel Hygiene Considerations
HACCP’s foundation is an effective prerequisite program based on the effective implementation of GMPs. Personnel hygiene considerations generally include but are not limited to:
• Cleanliness
• Illnesses
• Sores/wounds
• Smoking, eating, drinking, chewing
• Protective clothing
• Jewelry
• Training
Cleanliness
Workers should practice good personal hygiene habits including frequent bathing or showering. Excessive make-up, perfumes, colognes, aftershaves, false eyelashes, fake fingernails, and hair extensions should be prohibited in storage areas, laboratories, and inspection areas, and on the production floor. Frequent handwashing should be encouraged, and handwashing before and after eating, smoking, and vaping — as well as after using the rest room — should be required.
Illnesses
Any person with an apparent illness should not come into indirect or direct contact with raw materials, parts, components, assemblies, finished goods, tooling, molds, fixtures, gages, machines, or equipment to prevent contamination. Workers with an apparent illness should be required to immediately notify their supervisor. The best approach is to assign the worker other responsibilities until the illness has subsided. Worker should also be trained to cover coughs and sneezes to prevent contamination and spread of the illness.
Sores/Wounds
Any person with sores, wounds, boils, lacerations, skin infections, burns, abrasions, or lesions should not come into indirect or direct contact with raw materials, parts, components, assemblies, finished goods, tooling, molds, fixtures, gages, machines, or equipment to prevent contamination. Workers with sores, wounds, boils, lacerations, skin infections, burns, abrasions, or lesions should appropriately cover them. If the sores, wounds, abrasions, or lesions are on the hand, blue visible bandages and metal detectable bandages should be used. The employee should also be required to wear single-use gloves to ensure the bandage is covered and not inadvertently lost. Latex gloves should preferably be replaced with gloves made from nitrile or other inert materials because of the allergen risk associated with latex.
Smoking, Eating, Drinking, Chewing
Food, drinks, and medicines should not be allowed in storage areas, laboratories, or inspection areas, or on the production floor, to prevent contamination. With the recent rise of vaping as an alternative to smoking, GMP personnel hygiene procedures should explicitly prohibit vaping, in addition to smoking.

Figure 3: Vaping should be explicitly prohibited in personnel hygiene procedures
Food should be prohibited, not only to prevent contamination, but to keep potential allergens — especially from milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat and soybeans — out of storage areas, laboratories, inspection areas, and the production floor.
When organizations allow workers to drink in storage areas, laboratories, or inspection areas, or on the production floor, they must drink from approved containers (cups or water bottles with lids and straw). Generally, though, it is best to strictly prohibit drinking in GMP areas.
Workers with certain medical conditions should be allowed to keep their medications with them at all times (e.g., asthma inhalers).
Protective Clothing
Workers’ outer clothing should be clean, in good repair, and free from holes, sequins, and fur or faux fur. When required, hair nets and beard nets should be appropriately donned. If necessary, frocks, smocks, and lab coats should be changed when they become soiled, and they should not be worn into restrooms. Additionally, no objects should be attached to clothing or held in pockets above the waist. Of course, special considerations must be made for employees working under controlled environmental conditions such as a clean room.
Jewelry
All jewelry with the exception of medical alert bracelets or necklaces should be prohibited in storage areas, laboratories, and inspection areas, and on the production floor, including rings, watches, bracelets, necklaces, earrings, and exposed body piercings. The use of hairclips, and headbands should be prohibited.
Training
Personnel hygiene training should be required and provided for all full-time, part-time, temporary, and seasonal employees at hire and at least annually thereafter. Additionally, personnel hygiene training should be required and provided for all contractors and visitors. Records of all training for full-time, part-time, temporary, and seasonal employees, as well as for all contractors and visitors, should be retained.
Conclusion
Many firms successfully implement a personnel hygiene program but fail to review and adapt the program to address the ever-changing regulatory environment, as well other changes that can have negatively affect the safety of medicinal products. Implementing, maintaining, and sustaining a robust and effective personnel hygiene program to prevent contamination and cross contamination should be based upon an organization’s risk acceptance determination threshold, industry practice, guidance documents, and regulatory requirements.
References:
1. 21 CFR Part 820 Quality System Regulation, US FDA – current version.
2. 21 CFR Part 211 Current Good Manufacturing Practice for Finished
Pharmaceuticals, US FDA – current version.
3. ICH Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients,
ICH Harmonised Tripartite Guideline, 10 November 2000.
About The Author:
 Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC and is an ASQ Fellow and SRE Fellow. He earned a B.A.S. in computer-aided machining from Siena Heights University and an M.S. in quality management from Eastern Michigan University. He holds several certifications including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com with any questions or comments, and connect with him on LinkedIn.
Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC and is an ASQ Fellow and SRE Fellow. He earned a B.A.S. in computer-aided machining from Siena Heights University and an M.S. in quality management from Eastern Michigan University. He holds several certifications including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com with any questions or comments, and connect with him on LinkedIn.
Article originally published on April 17, 2019, in Pharmaceutical Online

